Abstract
To determine the baseline threat of microplastics and polycyclic aromatic hydrocarbons (PAHs) in an important seafood fish from Vueti Navakavu locally managed marine area, a multibiomarker risk assessment was conducted on the thumbprint emperor fish Lethrinus harak. Condition factor, a measure of relative general health condition of fish, was significantly lower in samples from the wet season compared to the dry season but no significant differences were observed for hepatosomatic index, a measure of relative stored energy/nutrition, between seasonal groups. PAHs levels of four metabolites in emperor fish from Fiji waters are reported here for the first time; seasonal groups showed no significant differences, but all samples presented levels of biliary PAHs. Each specimen also contained at least one microplastic in its gastrointestinal system; fibres were the predominant form-type and ingestion levels showed that more than 80% of fragment sizes were below 1.0 mm. Biochemical responses were observed for ethoxyresorufin-O-deethylase and glutathione S-transferase biotransformation activity, oxidative stress (glutathione peroxidase and glutathione reductase activity; lipid peroxidation) and genotoxicity (micronuclei assay). Though there were no statistically significant differences found, there were biological significances that were important to note; relatively low levels of pollutant exposure and low levels of biochemical responses showed enzymes response in thumbprint emperor were as expected to their roles in the body. In this multibiomarker approach, the observation of pollutants presence and histopathological injuries are considered biologically relevant from a toxicological perspective and serve as a baseline for future pollution studies in seafood fishes in Fiji, with site differences and the inclusion of fish species comparison. We recommend adopting a suite of biomarkers in future regional biomonitoring studies to develop holistic baseline information for other marine settings in Fiji and other Pacific Island countries.
Introduction
Marine pollution, in its many forms, poses a major threat to ocean life1,2. Pollutants in the marine environment are human-introduced chemicals or organically-sourced compounds that can influence the natural function or role of ecosystems and its inhabitants3. Two ubiquitous pollutants are microplastics (MPs) and polycyclic aromatic hydrocarbons (PAHs)4,5. MPs are described as plastic particles that have a size range between 0.01 and 5 mm6,7, while PAHs are organic compounds made of carbon and hydrogen, grouped into multiple aromatic rings and are primarily generated by incomplete combustion of organic materials8. MPs can spread across vast spaces of the ocean via currents and winds9, and are subject to progressive fragmentation due to mechanical abrasion, ultraviolet radiation, and biodegradation10. MPs transfer through the trophic food chain, bioaccumulating and biomagnifying in seafood, likely posing a risk to human health11,12. Specifically, the major route of human exposure to MPs is ingestion, which can lead to inflammatory lesions and immune disorders13,14. In Fiji, recent screenings have found MPs in seawater, sediments, and marine fishes15,16,17, while PAHs have not been studied yet18. However, studies elsewhere have found PAHs in different marine compartments like sediments19,20, water21,22 and biota23,24. PAHs represent a class of chemicals whose metabolites can exhibit toxicity even at low levels of exposure25; they bioaccumulate in marine bivalves, crustaceans and fishes26, and are subject to biomagnification in the food chain23,27. Some PAH metabolites are carcinogenic to humans28. PAHs are of interest for Pacific Island countries and are a priority area in the Pacific Regional Waste and Pollution Management Strategy 2016–2025, though no baseline data of these pollutants in Pacific Islands marine environment is available18. There is also a growing concern of MPs in Pacific Island seawaters, as highlighted by the Environmental Investigation Agency29 in 2020. MPs are recognised as a priority to address in the 2050 strategy for a Blue Pacific Continent.
The process of determining the presence or stages of effects of pollutants, like MPs and PAHs, in the environment or its inhabitants, is called environmental risk assessment30. Environmental risk assessments entail two approaches; environmental monitoring via chemistry surveillance31 and biomonitoring using biomarkers32. In Fiji, environmental monitoring has been used in some forms of environmental risk assessments, however, biomonitoring has yet to be applied18. The application of biomarkers in biomonitoring is useful for measuring a biochemical response of an animal when a pollutant causes a change to its biological state33. In general, these biochemical changes are responses occurring at the lower organismic levels; i.e., molecular, subcellular, cellular, histological34. Several biomarkers cover a range of measurable parameters for determining biological responses to marine pollution. For example, fish health can be evaluated with Fulton’s condition factor (K)35 and the hepatosomatic index (HSI), which are relative indications of general nutritional status and stored energy, respectively36. Both the K and the HSI of marine fishes are influenced by pollution exposure37—in particular, PAHs and MPs have been found to cause reduced K and HSI of marine fishes38. At the systemic level, there are biomarkers used to measure the activity of biotransformation enzymes. This includes cytochrome P450 enzyme such as the ethoxyresorufin O-deethylase (EROD) in phase I and the glutathione S-transferase (GST) in phase II39. Additionally, oxidative stress responses are measurable through antioxidant enzymes activity like the glutathione peroxidases (GPX)40, glutathione reductases (GR)41 and associated oxidative damages like lipid peroxidation (LPO)42. At the molecular level, the degradation of DNA integrity due to pollutants can be evaluated via the number of occurrences of deformed erythrocyte nucleotides as biomarkers of genotoxicity43.
Animals that are specifically selected for biomonitoring of pollutants in the environment are called sentinel species44. In particular, sentinel species are those animals that have measurable responses to the (class of) agents in question, including having a known habitat that overlaps the monitored area, are easily enumerated and captured, and have sufficient population size/density45. Some examples of marine sentinel species are the common periwinkle Littorina littorea46, the goose barnacle Pollicipes pollicipes47, the guri sea catfish Genidens genidens48, and the flathead grey mullet Mugil cephalus49. Continuous biological surveillance using sentinel species can be used to evaluate the effects of pollution in the marine environment50, but also to monitor the results of conservation and pollution mitigation efforts.
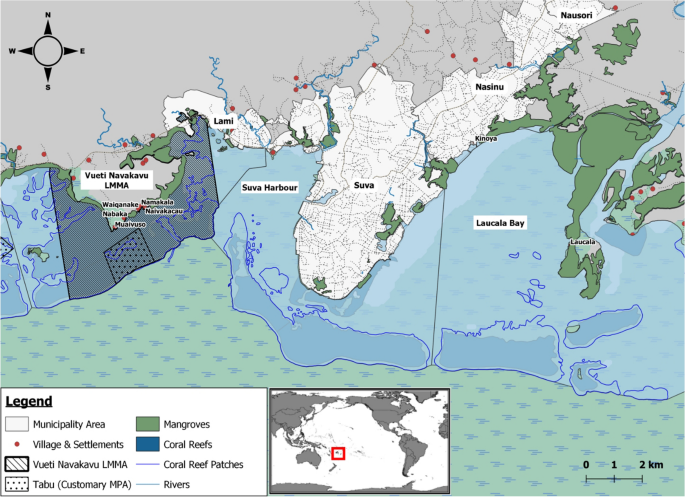
In Fiji, the customary systems of managing inshore marine resources include a prohibition of fishing (tabu) that can be declared by local clans or villages (mataqali) over the area (iqoliqoli), where a village exerts its customary fishing rights51. Traditionally, this type of prohibition is used in special circumstances, such as the death of a chief, and for a few months only52. However, in recent times, tabu has been used also by customary resource owners to protect and preserve their local marine resources. About 465 tabu areas, covering ca. 1000 km2, are part of the Fiji locally managed marine area (LMMA) network53. Among them, is the 19.1 km2 “Vueti Navakavu” LMMA, which lies 5 km west from the capital of Fiji, Suva, and is managed by the clan of Navakavu (which is spread over the three villages Muaivuso, Nabaka, and Waiqanake, and the two settlements Naivakacau and Namakala) (Fig. 1). About 20% (3.8 km2) of the Vueti Navakavu LMMA is a no-take tabu zone. Vueti Navakavu LMMA was designated in 2002 after the villagers identified several threats affecting the area, among which chemical and solid waste pollution were mentioned54. Despite the conservation effort, MPs pollution has been found in the locally managed marine area15,16. In addition, ocean surface currents, influenced by the southeast tradewinds55, might have brought in pollutants from the close-by Suva Harbour, where the presence of MPs and heavy metals, for example, has already been reported15,16,56.
Vueti Navakavu locally managed marine area (LMMA) and its customary marine protected area (tabu) in Viti Levu, Fiji. Inset: location of Fiji within the Pacific Ocean. Maps produced with QGIS Development Team57; maritime boundaries from the Secretariat of the Pacific Regional Environment Programme58—PacGeo network.
This study applies a biomonitoring ecotoxicological approach to environmental risk assessment in Vueti Navakavu LMMA in Fiji. Herein, PAHs exposure and presence, and abundance of MPs are quantified in the sentinel species thumbprint emperor (Lethrinus harak). Seasonality was considered when investigating the biological status of the thumbprint emperor to establish whether natural variation may influence pollution load into the marine environment, and thus affect the species interactions within (or changes to) the habitat.
Methods
Biological sampling
Sampling was performed from 100 to 250 m from the tabu area of Vueti Navakavu LMMA (Fig. 1) in April and July of 2017 and April and September of 2018, and it was performed to cover both the wet (November to April) and dry (May to October) tropical seasons. The thumbprint emperor was captured by local fishers with hook-and-line fishing gear. The live fish were placed in an 80 L portable tank filled with water from the fishing ground. Aeration was ensured by two submersible pumps (RS Electrical YS-702). In the village, the total weight and total length of each live fish were recorded using an analytical balance scale (precision: 0.1 g) and a measuring board (precision: 0.1 mm), respectively. Blood was extracted from the caudal vein of the live fish using a 21-gauge needle syringe and smeared onto a microscope glass slide to count for erythrocyte micronuclei formations43. The ethical sacrifice of the fish was then done by anaesthetising the fish in ice for 2 min, before severing a section in the vertebrae between the operculum and ray of the anterior dorsal fin using a scalpel blade59. The bile was extracted from the gall bladder using an insulin syringe for the fluorescence aromatic compounds analysis, then kept on ice until storage in a − 20 °C freezer. The liver was extracted and weighed. Five random sections of the liver were separated for the biochemical parameters and stored in liquid nitrogen until storage in a − 80 °C freezer.
Biomarkers
Fulton’s condition factor was calculated as K = total weight/length3 × 100. The hepatosomatic index was calculated as HSI = liver weight/total weight × 100. The PAH metabolites were determined through fixed wavelength fluorescence (FF) screening method60 and achieved by diluting the bile (10:1000 µL) in 48% ethanol before being measured spectrofluorometrically (absorbance and fluorescence intensity; double monochromotors) in a multimode reader (Thermo Scientific™ Varioskan™ MIB#5250030) to determine the signals intensity ratios of four biliary PAH metabolite types; phenanthrene (FF260/380), naphthalene (FF290/335), 1-hydroxypyrene (FF341/383), and benzo[a]pyrene (FF380/430)61,62. The multimode instrument reader measured at a dynamic wavelength range (emission: 200–1000 nm; excitation 5 nm and 12 nm/12 nm) with an accuracy of 0.003 Abs or ± 2%, at 200–399 nm (0–2 Abs) and 0.003 Abs or ± 1%, at 400–1000 nm (0–3 Abs), which was within the required spectrofluorometric parameters for the fluorescent aromatic compounds (FACs) analysis63. The quality assurance and quality control for the four biliary PAH metabolites included analytical standards for each of the PAH metabolites measured, calibration curves, continuing calibration standards, and method blanks in accordance with the technical guidelines described by the International Council for the Exploration of the Sea60,64. To assess the activity of biochemical analysis of EROD, the liver was homogenized in ice-cold buffer (50 mM Tris–HCL, pH 7.4, 0.15 M KCl)65. The S9 fraction of the hepatic tissue was homogenized66. The EROD activity was evaluated fluorometrically67. GST activity was determined by a substrate artificial 1-chloro-2, 4 dinitrobenzene, which was conjugated by GST68. GPX activity was determined through the metabolism of H2O2 to water, involving concomitant oxidation of reduced glutathione to its oxidized form glutathione disulfide63. GR activity was measured by catalysing the transformation of GSSG to reduced GSH with the concomitant oxidation of NADPH to NADP+ 69. The glutathione group of enzymes were measured at an absorbance of 340 nm. The peroxidative damage to lipids that occurs with reactive oxygen species generation and results in the production of malondialdehyde was assessed by the determination of TBARS. Malondialdehyde was determined by the thiobarbituric acid and was measured at an absorbance of 532 nm70. Micronuclei assay was assessed for genotoxicity. The blood smears were treated with methanol for 10 min to fix the cells and left to dry before staining cells with 5% Giemsa for 30 min. Determination of genotoxicity accounted for the total micronuclei occurrences recorded for 2000 erythrocytes per fish specimen71.
Microplastics
Extraction techniques for the removal of MPs from the gastrointestinal system (foregut to the hindgut) were done following Avio et al.72. In summary, a NaCl hypersaline (1.2 g/cm3) solution was added at three times the volume of the gastrointestinal system and was stirred and decanted twice for ten minutes, followed by filtration through a 63 µm sieve. The filtrate was transferred from the sieve into a 500 mL beaker and digestion of the suspended organic matter was performed by adding 30 mL of hydrogen peroxide (15%) to the sample before incubation at 60 °C for 24 h. The dried samples were resuspended with distilled water and filtered again through 63 µm sieve. Blank samples were analysed concomitantly with field samples, to gauge any potential cross-contamination. Identification of all plastic particles (different sizes and form type) was performed under a dissecting microscope (Olympus SZ-ST) at 10× magnification and an Infinity 1 camera whose images were processed through the Infinity Analyse software. All MPs larger than 0.5 mm were classified into fragment, film, fibre, and microbead based on their visual aspect73.
Statistical analyses
Seasonal differences in K, HSI, PAH metabolites and MPs were tested using non-parametric Wilcoxon-Mann–Whitney (WMW) test at a 5% significant level. All statistical tests were performed using the R software74.
Ethical statement
Fish specimens and data were collected according to the procedures and protocols approved by the University Research Ethics Committee (UREC) of the University of the South Pacific, in accordance with the policies and guideline stipulated in the Animal Research Ethics Handbook75. The processes and procedures of live specimen handling and field transportations were in compliance with Fiji’s regulation of animal protection as outlined in the Protection of Animals Act 195476. Euthanasia method on specimens was consistent with the commonly accepted norms of veterinary best practice77.
Results
A total of 53 specimens of thumbprint emperor were caught and sampled; 31 from the dry season and 22 from the wet season. Mean total weight was 185.5 g (SE = 12.9, range = 86.1–305.5 g) in the dry season and 212.9 g (SE = 13.2, range = 139.6–315.8 g) in the wet season. Average total length was 22.1 cm (SE = 0.7, range = 17.0–27.7 cm) in the dry season and 24.4 cm (SE = 0.5, range = 21.0–28.6 cm) in the wet season. The yearly average total weight was 196.9 g (SE = 9.5, range = 86.1–315.8 g), while the average total length was 23.1 cm (SE = 0.5, range = 17.0–28.6 cm).
Biomarkers
K was significantly lower (WMW test: Z = 2.02, p = 0.042) in samples from the wet season (mean ± SE = 1.42 ± 0.03) compared to the dry season (1.65 ± 0.04), while there was no significant difference in HSI (dry season = 0.82 ± 0.12; wet season = 0.47 ± 0.06; WMW test: Z = 1.21, p = 0.228). PAHs values (Table 1) were not significantly different among the two seasons (WMW test: benzo[a]pyrene: Z = − 1.04, p = 0.303; 1-hydroxypyrene: Z = 0.40, p = 0.689; phenanthrene Z = 1.83, p = 0.066; naphthalene: Z = − 1.03, p = 0.308). The observed biochemical responses (Table 2) were also not significantly different between the two seasons (WMW tests: EROD: Z = 0.17, p = 0.865; GST: Z = − 0.41, p = 0.685; GPX: Z = − 0.34, p = 0.741; GR: Z = − 0.83, p = 0.414; LPO: Z = − 1.82, p = 0.680; micronuclei assay: Z = 0.71, p = 0.407).
Microplastics
A total of 206 MPs were found. All sampled fish contained at least one MP, with three fish having 10 to 18 MP (Figure S1). On average, the ingestion levels were not significantly higher in fish from the wet season compared to those from the dry season (mean ± SE = 4.7 ± 0.9 MP/fish, 3.3 ± 1.3 MP/fish, respectively; WMW test: Z = − 0.62, p = 0.537). The majority of the MP found was less than 1.0 mm in size (Table 3). In particular, MPs ranging 0.1 to 0.4 mm made up 20% of the samples in the dry season and 50% of the samples in the wet season, while MPs ranging 0.5 to 0.9 mm made up 60% of the samples in the dry season and 38% in the wet season.
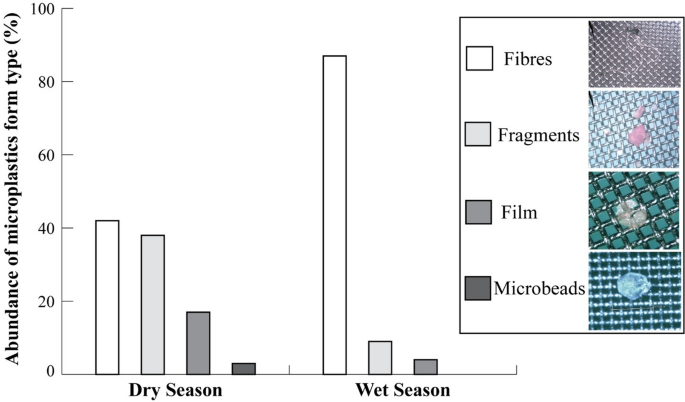
MP form type was dominated by fibres in the wet season (87% of 103 samples), while in the dry season a similar abundance of fibres and fragments (42% and 38%, respectively) was recorded (Fig. 2). Films had an overall lower abundance, although it was significantly higher in the dry season than in the wet season (0.6 ± 0.2 MP/fish and 0.2 ± 0.1 MP/fish, respectively; WMW test: Z = 2.38, p = 0.012). No significant difference was found in the abundance of fibres (dry season = 4.1 ± 0.9 MP/fish, wet season = 1.4 ± 0.3 MP/fish; WMW test: Z = − 2.01, p = 0.435) and fragments (dry season = 1.3 ± 0.2 MP/fish, wet season = 0.4 ± MP/fish; WMW test: Z = 1.73, p = 0.081) found in the fish. Microbeads had the lowest abundance and were found in the dry season only.
Discussion
This study demonstrates a low level of PAHs and MPs in the gastrointestinal tract of mature-sized thumbprint emperor of Vueti Navakavu LMMA in Fiji, South Pacific, both in the wet and dry seasons of the year. All the sampled specimens were of mature size78; the observed differences in K were thus most likely resulting from the reproductive activity. Indeed, the thumbprint emperor reportedly reproduces in the wet season79. This is further supported by a lower HSI in the wet season, suggesting a lower general stored energy. Variations in energy reserves based on the two physiological biomarkers can also occur as a result of recent feeding habits that can affect the liver size and bodyweight of the fish80. To identify whether reproduction or feeding behaviour resulted in the observed differences, we suggest identification of the maturation stage of the gonads via histology81 in future ecotoxicological studies.
The potential effects of PAHs on thumbprint emperor are of interest because the species, during its life cycle, occupies key positions in the food web82, and also because it is commonly consumed by the villagers of the local community83. PAHs enter fishes via food intake and during respiration, are then transported through the bloodstream, and adsorbed to lipid-rich tissues such as liver, muscle tissues, and gonads84. The concentration of PAHs found in fishes is normally higher than in the environment from which they were taken8. PAH metabolites were present in the bile of all thumbprint emperor sampled, ranging from 0.21 mg/L (benzo[a]pyrene type) to 75.74 mg/L (phenanthrene type) and, altogether, PAHs summed up to 127.10 mg/L. Compared with values found in the Atlantic cod (Gadus morhua) sampled from a PAH-polluted site in western Norway, where the lowest concentration was 66 mg/L (benzo[a]pyrene) and the highest concentration was 2704 mg/L (naphthalene)85, the ones found in the locally managed areas likely represent a low level of PAH pollution. However, coastal communities that tend to consume large quantities of fishes86 could be at greater risk to health issues like growth reduction, endocrine alterations87 and gastrointestinal infections88 due to PAH bioaccumulation and biomagnification. Monitoring of biliary biotransformation products permits detection of polar metabolites, which are proven to be sensitive PAH exposure markers, possibly two orders of magnitude more sensitive than tissue parent compound levels and is therefore ideal in monitoring and environmental risk assessment studies89. The majority of the fishes in Fijian rural areas are sourced locally from nearshore and coastal areas90 and in Vueti Navakavu, 88% of all households are involved in fishing activities for income and subsistence purposes91. Vethaak et al.92 found that marine areas close to industrial harbours have a gradual increase in PAH pollution over time. Site-specific pre-impact assessments are necessary to accurately evaluate before-after, control, impact (BACI) effects93. The values identified in this study represent a baseline against which comparison of future levels of biliary PAHs in the thumbprint emperor can be performed to assess environmental improvements in pollution reduction resulting from new policies and procedures.
The hepatic expressions for phase I and II biotransformation showed a total EROD and GST activity of 1.05 nmol/min/mg protein and 51.23 nmol/min/mg protein, respectively. A problem that frequently occurs in field studies with biomarkers is the difficulty of finding suitable reference values, which can be overcome with range-value comparisons of wild species to laboratory-conditioned species94. In this case, the Mozambique tilapia (Oreochromis mossambicus) exposed to phenanthrene concentration below 4.0 μg/g was found to have EROD activity of 5.4 nmol/min/mg protein, and to exhibit sublethal hepatotoxicity95. These biochemical values of EROD response are 5.1 times higher than those found in thumbprint emperor in this study, although without experimental studies in laboratorial conditions the sublethal consequences of this activity cannot be excluded. Compared with literature, GST activity in the present study was much lower than in two flatfishes (English sole Parophrys vetulus: 16-fold; starry flounder Platichthys stellatus: 44-fold) captured from a contaminated site in Puget Sound, Washington, and was related to higher activation and lower detoxification ability of PAHs resulting in hepatic neoplasms and putatively preneoplastic lesions96. Although the types of biotransformation reactions are similar between fishes, differences exist in the metabolic handling of chemicals; particularly reactions rates, the relative contribution of a given pathway, and the products formed97. From a functional viewpoint, biotransformation reactions can significantly influence the biological properties of chemicals, depending on the nature of the reaction and the rate at which it occurs98. In the case of the thumbprint emperor from Vueti Navakavu, phase I and II biotransformation activity were observed although there was no statistically clear relationship between the two pollutants.
Total activity for GPX and GR responses were 10.90 and 25.97 nmol/min/mg protein, respectively. The reaction of biomarkers GPX and GR is naturally inverse within a biological system69. The GPX responses in thumbprint emperor (10.90 nmol/min/mg protein) in the present study was lower than the one (11.89 nmol/min/mg protein) found on sterlet sturgeon (Acipenser ruthenus) in a petroleum-polluted site in Novi Sad (Serbia) but higher than the levels reported in a control site (10.30 nmol/min/mg protein) in the same place99. Consistently with the expected opposite enzymatic role of GR, the recorded GR activity in thumbprint emperor was 2.4 times higher than the GPX reaction. This is consistent with effective hepatic biochemical responses of the thumbprint emperor under oxidative stress due to oxidative-inducing pollutants, likely as a result of a significant accumulation of hydrogen peroxide100. We suggest including variations of antioxidant system response and its regulatory mechanisms under different circumstances (e.g., biological effects from exposure to heavy metals) in further studies, which would provide a valuable assessment of seafood fish quality and health risks from consumption.
A low level of biological damages was identified by LPO concentrations, whereby the levels found in the thumbprint emperor in the present study were 17 times lower than those found in spotted snakehead (Channa punctatus) in Aligarh, India, which were exposed to wastewater and, as a result, reported clear membrane damage. LPO concentrations mainly depend on the availability of polyunsaturated fatty acids and the antioxidants defences101. Consistently, the micronuclei assay in thumbprint emperor showed the very low occurrence of abnormal nuclei formation in erythrocytes (0.03%) compared to genotoxic findings on Nile tilapia (Oreochromis niloticus) (0.95%) captured from a heavy metals-polluted site in India102.
MPs were found in thumbprint emperors in both seasons, and the most abundant were smaller than 1.0 mm. The size of MPs may be a key factor in determining the range of animals that ingest them103, as well as the retention rate; for example, retention in the gastrointestinal system of spiny Chromis (Acanthochromis polyacanthus) increased (with a maximum of 2102 small particles) when the MPs size was reduced from 2 mm to 0.3–0.125 mm104. MPs cause oxidative damages in gills, muscle, and an increase in neurotoxic responses of fishes105. Low exposure to MPs is supported by both the low numbers of MPs found in thumbprint emperor as well as the low level of oxidative stress identified in the present study. Studies of MPs in seafood fish106,107,108 have also found that some pollutants like PAHs have a greater affinity to MPs than to water, and as a result MPs ingestion by seafood fishes likely have equal exposure to PAH intake (including other chemical pollutants), leading to bioaccumulation and biomagnification. It is for this reason that MPs and PAHs is recommended herein to be included in monitoring programmes in Fiji and other Pacific Island countries. While this baseline study observes the presence of MPs and PAHs in the thumbprint emperor, it only serves as a reference point for future pollution studies in seafood fishes in Fiji, and does not report risks or make recommendations from a human health perspective.
The PAHs and MPs pollutants quantified in this study represent a baseline for Vueti Navakavu LMMA, and indeed despite not having statistical significance, the interpretation of biologically relevant effects and efforts should be carefully considered. It is generally more valuable in ecotoxicology studies to know about the magnitude and certainty of an effect as well as its probability to occur, to ultimately evaluate its biological relevance109. Gagnon and Hodson110 demonstrated that biomarkers may vary in proportion to the extent of exposure to pollutants, and that individual studies might discover different magnitudes and directions of biomarker responses according to the specific situation investigated. In this study, the use of ecotoxicological tools (i.e. biomarkers) allows for direct assessment of the health of the thumbprint emperor within the LMMA since this approach shifts the focus of the assessment from the agents (pollutants) in the environmental compartments to the target species (biological responses)111. The thumbprint emperor, as aforementioned, showed relatively low levels of PAHs exposure and MPs ingestion, including relatively low levels of biological damages shown by ENA and LPO concentrations. The biochemical responses of Phase I and II biotransformation, GPX and GR activity were also comparatively low to studies that were identified. As a baseline for pollutants quantification and biochemical responses, it is important to not exclude the prospect of Vueti Navakavu’s twenty-long years as an LMMA being of some consequence to the health conditions and low pollutants exposure in the thumbprint emperor. Furthermore, this baseline study does not assume a “normal” biological state being defined for the thumbprint emperor, and the observation of pollutants presence and histopathological injuries that were observed in the fish is considered biologically relevant from a toxicological perspective112. It is for this reason that future biomonitoring studies is encouraged to build on the baseline information identified herein and employ a suite of biomarkers to bridge knowledge gaps and enable environmental risk assessment programmes to strengthen conservation efforts in marine spaces.
Marine protected areas are legally designated areas where human activities are restricted or managed to ensure sustainability and avoid over-fishing and habitat destruction113. Enforcement and management are essential to ensure that marine protected areas fulfil their purpose and do not remain mere polygons on a chart. Moreover, the effectiveness of these areas in protecting the marine and coastal habitat, their resistance and resilience to pollution, and their capacity of providing benefits like ecosystem services and ecological spillover, is related to the size of the MPAs and the place where the MPAs are established114.
The Vueti Navakavu LMMA is representative of Fiji’s key coral reef and mangrove coastal habitats, but it is relatively small-sized and its location is a few kilometres on the west of the harbour. Reportedly, the LMMA is threatened by chemical and solid waste pollution from the former Lami Dump and Suva city, both of which are located upwind and up-current115. It is important noting that the boundaries of the LMMA have cultural significance and are not ecological boundaries, therefore they are neutral and completely permeable to pollution.
Our study shows the presence of PAHs and MPs in all samples of emperor fish, an important seafood fish. Seasonal differences to the multibiomarker responses and pollutants levels were not statistically significant, which suggests that the threat posed by PAHs and MPs in Vueti Navakavu LMMA is all year round. Emerging pollutants (like MPs) and legacy pollutants (like PAHs) move unrestrictedly beyond conservation systems and boundaries such as LMMAs and MPAs. The baseline biological and pollutants parameters, herein, may provide meaningful insights for future biomonitoring studies in Fiji, with site differences and the inclusion of fish species comparison. We also recommend expanding the range and suite of biomarkers to contribute to enhancing a more holistic baseline information for other marine settings in Fiji and Pacific Island countries.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
- 1.
Ostiategui-Francia, P., Usategui-Martín, A. & Liria-Loza, A. Microplastics presence in sea turtles. In Fate and Impact of Microplastics in Marine Ecosystems (eds Baztan, J. et al.) 34–35 (Elsevier, 2017). https://doi.org/10.1016/B978-0-12-812271-6.00035-1.
- 2.
Wowk, K. M. Chapter 12—Paths to sustainable ocean resources. In Managing Ocean Environments in a Changing Climate (eds Noone, K. J. et al.) 301–348 (Elsevier, 2013). https://doi.org/10.1016/B978-0-12-407668-6.00012-4.
- 3.
Beiras, R. Chapter 16—Biological tools for monitoring: Biomarkers and bioassays. In Marine Pollution (ed. Beiras, R.) 265–291 (Elsevier, 2018). https://doi.org/10.1016/B978-0-12-813736-9.00016-7.
- 4.
Chen, S.-C. & Liao, C.-M. Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci. Total Environ. 366, 112–123 (2006).
- 5.
Zhang, Q. et al. A review of microplastics in table salt, drinking water, and air: Direct human exposure. Environ. Sci. Technol. 54, 3740–3751 (2020).
- 6.
Carpenter, E. J., Anderson, S. J., Harvey, G. R., Miklas, H. P. & Peck, B. B. Polystyrene spherules in coastal waters. Science 178, 749–750 (1972).
- 7.
Carpenter, E. J. & Smith, K. L. Plastics on the Sargasso sea surface. Science 175, 1240–1241 (1972).
- 8.
Abdel-Shafy, H. I. & Mansour, M. S. M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 25, 107–123 (2016).
- 9.
Wichmann, D., Delandmeter, P. & van Sebille, E. Influence of near-surface currents on the global dispersal of marine microplastic. J. Geophys. Res. Oceans 124, 6086–6096 (2019).
- 10.
Wang, W., Gao, H., Jin, S., Li, R. & Na, G. The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicol. Environ. Saf. 173, 110–117 (2019).
- 11.
Hantoro, I., Löhr, A. J., Van Belleghem, F. G. A. J., Widianarko, B. & Ragas, A. M. J. Microplastics in coastal areas and seafood: Implications for food safety. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 36, 674–711 (2019).
- 12.
Mercogliano, R. et al. Occurrence of microplastics in commercial seafood under the perspective of the human food chain. A review. J. Agric. Food Chem. 68, 5296–5301 (2020).
- 13.
Cox, K. D. et al. Human consumption of microplastics. Environ. Sci. Technol. 53, 7068–7074 (2019).
- 14.
Prata, J. C., da Costa, J. P., Lopes, I., Duarte, A. C. & Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 702, 134455 (2020).
- 15.
Dehm, J., Singh, S., Ferreira, M. & Piovano, S. Microplastics in subsurface coastal waters along the southern coast of Viti Levu in Fiji, South Pacific. Mar. Pollut. Bull. 156, 111239 (2020).
- 16.
Ferreira, M., Thompson, J., Paris, A., Rohindra, D. & Rico, C. Presence of microplastics in water, sediments and fish species in an urban coastal environment of Fiji, a Pacific small island developing state. Mar. Pollut. Bull. 153, 110991 (2020).
- 17.
Reisser, J. et al. Marine plastic pollution in waters around Australia: Characteristics, concentrations, and pathways. PLoS ONE 8, e80466 (2013).
- 18.
Varea, R., Piovano, S. & Ferreira, M. Knowledge gaps in ecotoxicology studies of marine environments in Pacific Island Countries and Territories—A systematic review. Mar. Pollut. Bull. 156, 111264 (2020).
- 19.
Yang, X., Yu, L., Chen, Z. & Xu, M. Bioavailability of polycyclic aromatic hydrocarbons and their potential application in eco-risk assessment and source apportionment in urban river sediment. Sci. Rep. 6, 23134 (2016).
- 20.
Girardin, V., Grung, M. & Meland, S. Polycyclic aromatic hydrocarbons: Bioaccumulation in dragonfly nymphs (Anisoptera), and determination of alkylated forms in sediment for an improved environmental assessment. Sci. Rep. 10, 10958 (2020).
- 21.
Sarria-Villa, R., Ocampo-Duque, W., Páez, M. & Schuhmacher, M. Presence of PAHs in water and sediments of the Colombian Cauca River during heavy rain episodes, and implications for risk assessment. Sci. Total Environ. 540, 455–465 (2016).
- 22.
Witt, G. Polycyclic aromatic hydrocarbons in water and sediment of the Baltic Sea. Mar. Pollut. Bull. 31, 237–248 (1995).
- 23.
Rosenfeld, E. & Feng, H. Bioaccumulation of dioxins, PCBs, and PAHs. In Risks of Hazardous Wastes (eds Rosenfeld, P. E. & Feng, L.) 201–213 (Elsevier, 2011).
- 24.
Shukla, S. K., Mangwani, N., Rao, T. S. & Das, S. 8—Biofilm-mediated bioremediation of polycyclic aromatic hydrocarbons. In Microbial Biodegradation and Bioremediation (ed. Das, S.) 203–232 (Elsevier, 2014). https://doi.org/10.1016/B978-0-12-800021-2.00008-X.
- 25.
Crawford, C. & Quinn, B. The interactions of microplastics and chemical pollutants. In Microplastic Pollutants (eds Crawford, C. B. & Quinn, B.) 131–157 (Elsevier, 2017).
- 26.
Law, K. L. & Thompson, R. C. Microplastics in the seas. Science 345, 144–145 (2014).
- 27.
Wang, D.-Q. et al. Polycyclic aromatic hydrocarbons and organochlorine pesticides in fish from Taihu Lake: Their levels, sources, and biomagnification. Ecotoxicol. Environ. Saf. 82, 63–70 (2012).
- 28.
International Agency for the Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures (IARC, 2010).
- 29.
Farrelly, T., Borrelle, S. & Fuller, S. Plastic Pollution Prevention in Pacific Islands: Gap Analysis of Current Legislation, Policies and Plans (Environment Investigation Agency-International, 2020).
- 30.
Jager, T. Dynamic modelling for uptake and effects of chemicals. In Marine Ecotoxicology (eds Blasco, J. et al.) 71–98 (Elsevier, 2016). https://doi.org/10.1016/b978-0-12-803371-5.00003-5.
- 31.
Artiola, J. & Brusseau, M. The role of environmental monitoring in pollution science. In Environmental and Pollution Science (eds Pepper, I. L. et al.) 149–162 (Elsevier, 2019).
- 32.
Hoet, P. & Haufroid, V. Biological monitoring: State of the art. Occup. Environ. Med. 54, 361–366 (1997).
- 33.
Connon, R. E., Geist, J. & Werner, I. Effect-based tools for monitoring and predicting the ecotoxicological effects of chemicals in the aquatic environment. Sensors 12, 12741–12771 (2012).
- 34.
Lionetto, M. G., Caricato, R. & Giordano, M. E. Pollution biomarkers in environmental and human biomonitoring. Open Biomark. J. 9, 1–9 (2019).
- 35.
Nash, R. D., Valencia, A. H. & Geffen, A. J. The origin of Fulton’s condition factor—Setting the record straight. Fisheries 31, 236–238 (2006).
- 36.
Zhelev, Z. M., Popgeorgiev, G. S. & Mehterov, N. H. Changes in the hepatosomatic index and condition factor in the populations of Pelophylax ridibundus (Amphibia: Ranidae) from anthropogenically polluted biotopes in southern Bulgaria. Part II. Bulg. J. Agric. Sci. 3, 517–522 (2015).
- 37.
Araújo, F. G. et al. Biomarkers and bioindicators of the environmental condition using a fish species (Pimelodus maculatus Lacepède, 1803) in a tropical reservoir in Southeastern Brazil. Braz. J. Biol. 78, 351–359 (2018).
- 38.
Arias, A. H., Ronda, A. C., Oliva, A. L. & Marcovecchio, J. E. Evidence of microplastic ingestion by fish from the bahía Blanca Estuary in Argentina, South America. Bull. Environ. Contam. Toxicol. 102, 750–756 (2019).
- 39.
Li, M.-H. Development of in vivo biotransformation enzyme assays for ecotoxicity screening: In vivo measurement of phases I and II enzyme activities in freshwater planarians. Ecotoxicol. Environ. Saf. 130, 19–28 (2016).
- 40.
Gardiner, M., Thomas, T. & Egan, S. A glutathione peroxidase (GpoA) plays a role in the pathogenicity of Nautella italica strain R11 towards the red alga Delisea pulchra. FEMS Microbiol. Ecol. 91, fiv021 (2015).
- 41.
Al-Ghais, S. M. Acetylcholinesterase, glutathione and hepatosomatic index as potential biomarkers of sewage pollution and depuration in fish. Mar. Pollut. Bull. 74, 183–186 (2013).
- 42.
Bethanie, A. C. Oxidative Damage in Fish Used as Biomarkers in Field and Laboratory Studies (Department of Zoology/Zoophysiology, Göteborg University, 2008).
- 43.
Cavalcante, D. G. S. M., Martinez, C. B. R. & Sofia, S. H. Genotoxic effects of Roundup® on the fish Prochilodus lineatus. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 655, 41–46 (2008).
- 44.
Bossart, G. D. Marine mammals as sentinel species for oceans and human health. Vet. Pathol. 48, 676–690 (2011).
- 45.
National Research Council. Animals as Sentinels of Environmental Health Hazards (National Academies Press, 1991).
- 46.
Noventa, S., Pavoni, B. & Galloway, T. S. Periwinkle (Littorina littorea) as a sentinel species: A field study integrating chemical and biological analyses. Environ. Sci. Technol. 45, 2634–2640 (2011).
- 47.
Ramos, A. S., Antunes, S. C., Gonçalves, F. & Nunes, B. The Gooseneck barnacle (Pollicipes pollicipes) as a candidate sentinel species for coastal contamination. Arch. Environ. Contam. Toxicol. 66, 317–326 (2014).
- 48.
Silva Junior, D. R., Carvalho, D. M. T. & Vianna, M. The catfish Genidens genidens (Cuvier, 1829) as a potential sentinel species in Brazilian estuarine waters. J. Appl. Ichthyol. 29, 1297–1303 (2013).
- 49.
Ferreira, M., Antunes, P., Gil, O., Vale, C. & Reis-Henriques, M. A. Organochlorine contaminants in flounder (Platichthys flesus) and mullet (Mugil cephalus) from Douro estuary, and their use as sentinel species for environmental monitoring. Aquat. Toxicol. 69, 347–357 (2004).
- 50.
Eriksen, M. et al. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 9, e111913 (2014).
- 51.
Veitayaki, J. Fisheries Development in Fiji: The Quest for Sustainability (Institute of Pacific Studies, 1995).
- 52.
Veitayaki, J., Breckwoldt, A., Sigarua, T., Bulai, N. & Rokomate-Nakoro, A. Living from the Sea: Culture and Marine Conservation in Fiji (iTaukei Trust Fund Board, 2016).
- 53.
Damien. LMMA Fiji. The LMMA Network. https://lmmanetwork.org/who-we-are/country-networks/fiji/ (2016).
- 54.
UN WCMC ICCA Registry. The ICCA Registry. The ICCA Registry. https://www.iccaregistry.org/en/explore/Fiji/vueti-navakavu (2020).
- 55.
Kumar, V. V., Deo, R. C. & Ramachandran, V. Total rain accumulation and rain-rate analysis for small tropical Pacific islands: A case study of Suva, Fiji. Atmos. Sci. Lett. 7, 53–58 (2006).
- 56.
Maata, M. & Singh, S. Heavy metal pollution in Suva harbour sediments, Fiji. Environ. Chem. Lett. 6, 113–118 (2008).
- 57.
QGIS Development Team. QGIS Geographic Information System (QGIS Development Team, 2020).
- 58.
Secretariat of the Pacific Regional Environment Programme. GIS & Spatial Data Dashboard | Pacific Environment Portal. https://pacific-data.sprep.org/gis-spatial-data-dashboard (2019).
- 59.
Bennett, R. H. et al. Ethical considerations for field research on fishes. Koedoe 58, 1–15 (2016).
- 60.
International Council for the Exploration of the Sea. ICES Techniques in Marine Environmental Sciences (ICES, 1996).
- 61.
Struch, R. E., Pulster, E. L., Schreier, A. D. & Murawski, S. A. Hepatobiliary analyses suggest chronic PAH exposure in hakes (Urophycis spp.) following the Deepwater Horizon oil spill. Environ. Toxicol. Chem. 38, 2740–2749 (2019).
- 62.
Pulster, E. L. et al. Chronic PAH exposures and associated declines in fish health indices observed for ten grouper species in the Gulf of Mexico. Sci. Total Environ. 703, 135551 (2020).
- 63.
Ferreira, M., Moradas-Ferreira, P. & Reis-Henriques, M. A. The effect of long-term depuration on levels of oxidative stress biomarkers in mullets (Mugil cephalus) chronically exposed to contaminants. Mar. Environ. Res. 64, 181–190 (2007).
- 64.
Ariese, F., Beyer, J., Jonsson, G., Visa, C. & Krahn, M. M. ICES Techniques in Marine Environmental Sciences 41 (ICES, 2005). https://doi.org/10.25607/obp-225.
- 65.
Whyte, J. J., Jung, R. E., Schmitt, C. J. & Tillitt, D. E. Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit. Rev. Toxicol. 30, 347–570 (2000).
- 66.
Fent, K. & Bucheli, T. D. Inhibition of hepatic microsomal monooxygenase system by organotins in vitro in freshwater fish. Aquat. Toxicol. 28, 107–126 (1994).
- 67.
Ferreira, M. et al. Assessment of contaminants and biomarkers of exposure in wild and farmed seabass. Ecotoxicol. Environ. Saf. 73, 579–588 (2010).
- 68.
Wang, Z., Jin, L., Wegrzyn, G. & Wegrzyn, A. A novel method for screening the glutathione transferase inhibitors. BMC Biochem. 10, 6–6 (2009).
- 69.
Ribalta, C., Sanchez-Hernandez, J. C. & Sole, M. Hepatic biotransformation and antioxidant enzyme activities in Mediterranean fish from different habitat depths. Sci. Total Environ. 532, 176–183 (2015).
- 70.
Moore, K. & Roberts, L. J. Measurement of lipid peroxidation. Free Radic. Res. 28, 659–671 (1998).
- 71.
Ayllon, F. & Garcia-Vazquez, E. Induction of micronuclei and other nuclear abnormalities in European minnow Phoxinus phoxinus and mollie Poecilia latipinna: An assessment of the fish micronucleus test. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 467, 177–186 (2000).
- 72.
Avio, C. G., Gorbi, S. & Regoli, F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar. Environ. Res. 111, 18–26 (2015).
- 73.
Rochman, C. M. et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 38, 703–711 (2019).
- 74.
RSoftware. RStudio: Integrated Development Environment for R (R Studio Incorporated, 2018).
- 75.
The University of the South Pacific. Animal Research Ethics: A Handbook for USP Researchers (The University of the South Pacific, 2020).
- 76.
Office of the Attorney General. Protection of Animals Act 1954. https://www.laws.gov.fj/Acts/DisplayAct/685#.
- 77.
American Veterinary Medical Association. Guidelines for the Euthanasia of Animals. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition (2020).
- 78.
Fishbase. Lethrinus harak, Thumbprint emperor: Fisheries. https://www.fishbase.se/summary/Lethrinus-harak.html (2020).
- 79.
Prince, J. et al. Spawning potential surveys in Fiji: A new song of change for small-scale fisheries in the Pacific. Conserv. Sci. Pract. 3, e273 (2020).
- 80.
Leatherhead, J. & Woo, P. Fish Diseases and Disorders. Volume 2: Non-infectious Disorders (CABI Publishing, Wallingford, 1998).
- 81.
Carducci, F. et al. Omics approaches for conservation biology research on the bivalve Chamelea gallina. Sci. Rep. 10, 19177 (2020).
- 82.
Carpenter, K. FAO species catalogue, volume 9. Emperor fishes and large-eye breams of the world (family Lethrinidae). FAO Fish Synopsis 9, 75–77 (1989).
- 83.
Prince, J. et al. Developing a system of sustainable minimum size limits for Fiji. South Pac. Bull. 155, 51–60 (2017).
- 84.
Logan, D. T. Perspective on ecotoxicology of PAHs to fish. Hum. Ecol. Risk Assess. Int. J. 13, 302–316 (2007).
- 85.
Aas, E., Beyer, J., Jonsson, G., Reichert, W. L. & Andersen, O. K. Evidence of uptake, biotransformation and DNA binding of polyaromatic hydrocarbons in Atlantic cod and corkwing wrasse caught in the vicinity of an aluminium works. Mar. Environ. Res. 52, 213–229 (2001).
- 86.
Wei, S. et al. Trace organic contamination in biota collected from the Pearl River Estuary, China: A preliminary risk assessment. Mar. Pollut. Bull. 52, 1682–1694 (2006).
- 87.
Meador, J. P., Sommers, F. C., Ylitalo, G. M. & Sloan, C. A. Altered growth and related physiological responses in juvenile Chinook salmon (Oncorhynchus tshawytscha) from dietary exposure to polycyclic aromatic hydrocarbons (PAHs). Can. J. Fish. Aquat. Sci. 63, 2364–2376 (2006).
- 88.
Soós, K. The occurrence of carcinogenic polycyclic hydrocarbons in foodstuffs in Hungary. In Further Studies in the Assessment of Toxic Actions (eds Chambers, P. L. & Klinger, W.) 446–448 (Springer, 1980). https://doi.org/10.1007/978-3-642-67729-8_104.
- 89.
Beyer, J., Jonsson, G., Porte, C., Krahn, M. M. & Ariese, F. Analytical methods for determining metabolites of polycyclic aromatic hydrocarbon (PAH) pollutants in fish bile: A review. Environ. Toxicol. Pharmacol. 30, 224–244 (2010).
- 90.
Glaus, K. B. J. et al. Fishing for profit or food? Socio-economic drivers and fishers’ attitudes towards sharks in Fiji. Mar. Policy 100, 249–257 (2019).
- 91.
Charlton, K. E. et al. Fish, food security and health in Pacific Island countries and territories: A systematic literature review. BMC Public Health 16, 285 (2016).
- 92.
Vethaak, A. D. & Leslie, H. A. Plastic debris is a human health issue. Environ. Sci. Technol. 50, 6825–6826 (2016).
- 93.
Pulster, E. L. et al. A first comprehensive baseline of hydrocarbon pollution in Gulf of Mexico fishes. Sci. Rep. 10, 6437 (2020).
- 94.
Monteiro, M. et al. Impact of chemical exposure on the fish Pomatoschistus microps Krøyer (1838) in estuaries of the Portuguese Northwest coast. Chemosphere 66, 514–522 (2007).
- 95.
Shailaja, M. S. & D’Silva, C. Evaluation of impact of PAH on a tropical fish, Oreochromis mossambicus using multiple biomarkers. Chemosphere 53, 835–841 (2003).
- 96.
Collier, T. K., Singh, S. V., Awasthi, Y. C. & Varanasi, U. Hepatic xenobiotic metabolizing enzymes in two species of benthic fish showing different prevalences of contaminant-associated liver neoplasms. Toxicol. Appl. Pharmacol. 113, 319–324 (1992).
- 97.
Tilson, H. A. EHP papers of the year 2008. Environ. Health Perspect. 116, A234 (2008).
- 98.
Lech, J. J. & Vodicnik, J. Biotransformation of chemicals by fish: An overview. In Cancer Mortality in the United States, 1950–1977 (ed. McKay, F. W.) 355 (National Cancer Institute, 1982).
- 99.
Stanic, B., Andric, N., Zoric, S., Grubor-Lajsic, G. & Kovacevic, R. Assessing pollution in the Danube River near Novi Sad (Serbia) using several biomarkers in sterlet (Acipenser ruthenus L.). Ecotoxicol. Environ. Saf. 65, 395–402 (2006).
- 100.
dos Carvalho, C. S., Bernusso, V. A., de Araújo, H. S. S., Espíndola, E. L. G. & Fernandes, M. N. Biomarker responses as indication of contaminant effects in Oreochromis niloticus. Chemosphere 89, 60–69 (2012).
- 101.
Javed, M., Ahmad, M. I., Usmani, N. & Ahmad, M. Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste water. Sci. Rep. 7, 1675 (2017).
- 102.
Khan, M. S., Javed, M., Rehman, M. T., Urooj, M. & Ahmad, M. I. Heavy metal pollution and risk assessment by the battery of toxicity tests. Sci. Rep. 10, 16593 (2020).
- 103.
Andrady, A. L. Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605 (2011).
- 104.
Critchell, K. & Hoogenboom, M. O. Effects of microplastic exposure on the body condition and behaviour of planktivorous reef fish (Acanthochromis polyacanthus). PLoS ONE 13, e0193308 (2018).
- 105.
Barboza, L. G. A. et al. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 717, 134625 (2020).
- 106.
Smith, M., Love, D. C., Rochman, C. M. & Neff, R. A. Microplastics in seafood and the implications for human health. Curr. Environ. Health Rep. 5, 375–386 (2018).
- 107.
Tien, C.-J., Wang, Z.-X. & Chen, C. S. Microplastics in water, sediment and fish from the Fengshan River system: Relationship to aquatic factors and accumulation of polycyclic aromatic hydrocarbons by fish. Environ. Pollut. 265, 114962 (2020).
- 108.
Pannetier, P. et al. Environmental samples of microplastics induce significant toxic effects in fish larvae. Environ. Int. 134, 105047 (2020).
- 109.
Feckler, A., Low, M., Zubrod, J. P. & Bundschuh, M. When significance becomes insignificant: Effect sizes and their uncertainties in Bayesian and frequentist frameworks as an alternative approach when analyzing ecotoxicological data. Environ. Toxicol. Chem. 37, 1949–1955 (2018).
- 110.
Gagnon, M. M. & Hodson, P. V. Field studies using fish biomarkers—How many fish are enough?. Mar. Pollut. Bull. 64, 2871–2876 (2012).
- 111.
Gusso-Choueri, P. K. et al. Assessing pollution in marine protected areas: The role of a multi-biomarker and multi-organ approach. Environ. Sci. Pollut. Res. 22, 18047–18065 (2015).
- 112.
EFSA Scientific Committee. Statistical significance and biological relevance. EFSA J. 9, 2372 (2011).
- 113.
Smallhorn-West, P. F., Weeks, R., Gurney, G. & Pressey, R. L. Ecological and socioeconomic impacts of marine protected areas in the South Pacific: Assessing the evidence base. Biodivers. Conserv. 29, 349–380 (2020).
- 114.
Gurney, G. G. et al. Efficient and equitable design of marine protected areas in Fiji through inclusion of stakeholder-specific objectives in conservation planning. Conserv. Biol. 29, 1378–1389 (2015).
- 115.
Thaman, R. R., Fong, T. & Balawa, A. Biodiversity and ethnobiodiversity of finfishes of Vanua Navakau, Viti Levu, Fiji Islands. vol. 4 54 https://spccfpstore1.blob.core.windows.net/digitallibrary-docs/files/88/88a1f351144fda547a2b74821378837f.pdf?sv=2015-12-11&sr=b&sig=7FE3M2PyLkAzBAobwSYT7tc%2BObPd6lOg7oXLcdmHzC4%3D&se=2020-04-03T23%3A45%3A50Z&sp=r&rscc=public%2C%20max-age%3D864000%2C%20max-stale%3D86400&rsct=application%2Fpdf&rscd=inline%3B%20filename%3D%22ENG_2008_Biodiversity_ethnodiversity_finfishes.pdf%22 (2008).
Acknowledgements
We acknowledge the community members of Vueti Navakavu LMMA for allowing and facilitating sampling in their community. We thank Simi Naivalu for his laboratory assistance in the identification of MPs. R.V. was supported by a USP-PEUMP programme scholarship (Grant No. F3289-FST41-71505-001), this research was funded by USP SRT (Grant No. F7102-RI001-ACC-001) to M.F and S.P.
Author information
Affiliations
Contributions
R.V.: conceptualization, data curation, funding acquisition, methodology, formal analysis, investigation, writing original draft. A.P.: formal analysis, data curation, investigation, writing—review and editing. M.F.: conceptualization, investigation, funding acquisition, supervision. S.P.: conceptualization, funding acquisition, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Varea, R., Paris, A., Ferreira, M. et al. Multibiomarker responses to polycyclic aromatic hydrocarbons and microplastics in thumbprint emperor Lethrinus harak from a South Pacific locally managed marine area. Sci Rep 11, 17991 (2021). https://ift.tt/3jXInBm
-
Received:
-
Accepted:
-
Published:
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
"aromatic" - Google News
September 09, 2021 at 04:46PM
https://ift.tt/3tthiJh
Multibiomarker responses to polycyclic aromatic hydrocarbons and microplastics in thumbprint emperor Lethrinus harak from a South Pacific locally managed marine area | Scientific Reports - Nature.com
"aromatic" - Google News
https://ift.tt/2A2dLdj
https://ift.tt/3b8aPsv
Bagikan Berita Ini

















0 Response to "Multibiomarker responses to polycyclic aromatic hydrocarbons and microplastics in thumbprint emperor Lethrinus harak from a South Pacific locally managed marine area | Scientific Reports - Nature.com"
Post a Comment