Peptides exhibit surprisingly strong diamagnetism when they join together to form microfibres, say researchers in China. Haijun Yang, at the Chinese Academy of Sciences (CAS) in Shanghai, and colleagues measured the force exerted by peptide-containing liquids placed in a static magnetic field. They found that samples in which the peptides had been allowed to self-assemble had a diamagnetic mass susceptibility 11 times higher than samples in which the peptides were dissolved, and 175 times that of pure water. The unexpected result, reported in Chinese Physics Letters, helps to explain the origin of magnetism in biomolecules, and could have applications in magnetically controlled microfabrication, medical imaging and brain–computer interfaces.
A diverse range of biological structures – from entire cells down to DNA and other subcellular polymers – are known to change their orientation in response to magnetic fields. This effect is behind the ability of some animals to navigate using the Earth’s magnetic field. However, many of the biological structures that respond to such fields apparently contain too little iron for the phenomenon to be explained by either ferromagnetism or ferrimagnetism. This suggests that the magnetic sensitivity of these structures is instead due to more subtle magnetic effects.
In an amino-acid chain called a peptide, for example, an external magnetic field induces a weak secondary field with the opposite direction, which pushes the peptide away. This effect, known as diamagnetism, has been attributed to the alignment of electrons in the peptide bonds between amino acids, or to electron currents flowing around cyclic structures called aromatic rings.
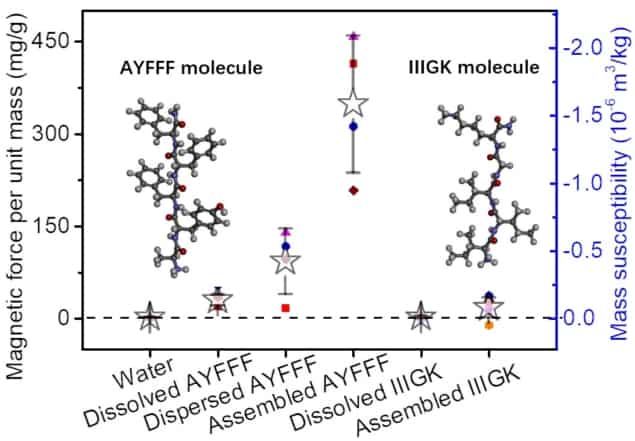
To investigate this phenomenon, Yang and colleagues prepared three different samples of a pentapeptide with the sequence AYFFF. A pentapeptide is a polymer consisting specifically of five amino acid units – in this case, one unit of alanine (A), one of tyrosine (Y) and three of phenylalanine (F).
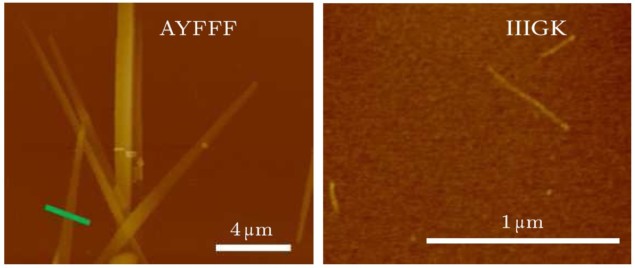
In the first sample, the researchers dissolved the peptide in the organic solvent dimethyl sulfoxide (DMSO). In the second sample, they dispersed the powdered peptide in water. In the third sample, they also dispersed the powdered peptide in water, but then left it to stand for 16 hours. Over this 16-hour period, the peptides self-assembled into fibres more than 10 µm long.
The researchers weighed the samples, and then repeated the measurement in the presence of a magnetic field, which they applied by placing a permanent magnet above the apparatus. They assumed that any difference between measurements with and without the magnet was due to the diamagnetic interaction between the external magnetic field and the magnetic fields induced within the sample.
They found that the peptide dissolved in DMSO exhibited the weakest diamagnetic response, while the sample in which the peptide had been allowed to self-assemble exhibited the strongest. The strength of diamagnetism in the water-dispersed sample was somewhere between the two, which the researchers put down to the occurrence of a limited degree of self-assembly.
When the team performed the same experiment using a different pentapeptide – IIIGK, comprising isoleucine (I), glycine (G) and lysine (K) – they found only a weak diamagnetic response, even for the sample that had been left to self-assemble into fibril structures. Whereas tyrosine and phenylalanine in the AYFFF sequence incorporate aromatic rings in their structures, this is not the case for any of the components of the IIIGK peptide. Yang and colleagues therefore interpret their results as confirmation that the electron dynamics of the aromatic rings, rather than the peptide bonds, are responsible for strong diamagnetism in peptides.
Although the role of aromatic ring currents was not unexpected in itself, the researchers were surprised by how strong the diamagnetism was in the self-assembled peptide samples. They are still investigating the physics underlying the effect, but they do already have an idea of how such anomalously strong diamagnetism might arise.
“Each peptide molecule has an induced magnetic moment in the presence of an applied magnetic field,” says Haiping Fang, of the CAS and East China University of Science and Technology in Shanghai, who led the research. “Thermal forces randomize the orientation of these magnetic moments, weakening the diamagnetism. A group of aromatic peptides in the assembled state – with interactions between them – might reduce the effect of these thermal fluctuations, resulting in the strong diamagnetism.”
Whatever its origin, the researchers foresee several possible applications for the effect. “Peptides with various sequences could be easily assembled into different nanostructures under magnetic fields, widening fabrication strategies,” says Feng Zhang, a collaborator from Guangzhou Medical University. “Assembled peptides modified with biomarkers could also be used as biological tracers, enhancing the contrast between normal and abnormal tissues in magnetic resonance imaging. Or, in novel brain–computer interfaces, the assembled peptides’ strong diamagnetism could be used as a non-invasive sensor to detect weak variations in magnetic signals from brain activity.”
"aromatic" - Google News
September 07, 2020 at 04:29PM
https://ift.tt/2R3v0Qw
Self-assembled peptides exhibit surprisingly strong diamagnetism - physicsworld.com
"aromatic" - Google News
https://ift.tt/2A2dLdj
https://ift.tt/3b8aPsv
Bagikan Berita Ini

















0 Response to "Self-assembled peptides exhibit surprisingly strong diamagnetism - physicsworld.com"
Post a Comment